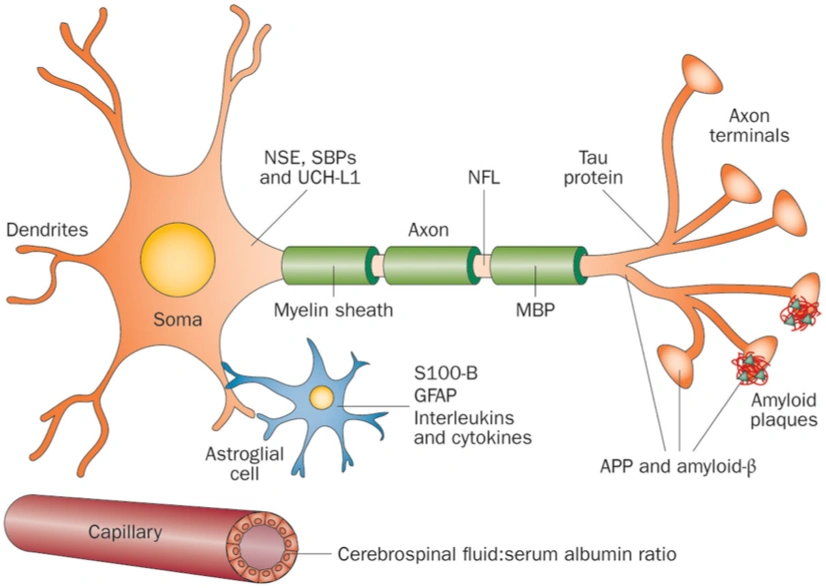
ASTROGLIAL CELL MARKERS
S100B
S100B is a calcium-binding peptide and is used as a parameter of glial activation and/or death in many disorders of the central nervous system (CNS). It plays important roles in normal CNS development and recovery after injury. Although S100B is mainly found in astroglial and Schwann cells, it also has extracerebral sources. S100B is a useful neurobiochemical marker of brain damage such as in circulatory arrest, stroke and traumatic brain injury. S100B is also associated with neurodegenerative diseases like Alzheimer's disease or other chronic neurological diseases. Moreover, S100B may have a potential in predicting the efficiency of treatment and prognosis.
Binding of calcium to S100B induces a large conformational change that allows hydrophobic residues to expose and interact with other proteins in order to confer biological activity.3. S100B is located in the cytoplasm and nucleus of the astrocytes, and it regulates the cytoskeletal structure and cell proliferation.4. Although it has been shown that S100B is mainly found in astroglial and Schwann cells, it has also been found in adipocytes, chondrocytes, lymphocytes, bone marrow cells, and melanocytes.5. S100B is mainly eliminated by the kidney.
The effects of S100B depend on its concentrations. At nanomolar concentrations, S100B in vitro stimulates neurite outgrowth in cerebral cortex neurons and enhances survival of neurons in various systems during development.5. S100B in vitro has a neurotrophic activity for neuronal cells during the neuronal maturation and glial cell proliferation. S100B decreases cell death and the loss of mitochondrial function resulting from glucose deprivation.1, 5 With its neurotrophic and gliotrophic actions, S100B probably plays important roles in normal CNS development and recovery after injury. In contrast to the stated effects of nanomolar levels of S100B, micromolar levels of extracellular S100B may have deleterious effects.6. At these concentrations, extracellular S100B in vitro stimulates the expression of proinflammatory cytokines and induces apoptosis.1 S100B exerts its neurotoxic effects in vitro by inducing apoptosis in neurons. Recent observations show that micromolar concentrations of S100B produce apoptotic death by interacting with the Receptor for Advanced Glycation End Products (RAGE), causing elevation in reactive oxygen species, cytochrome C release and activation of the caspase cascade.6. S100B might contribute to neuropathological changes in the course of neurodegeneration and/or brain inflammatory diseases by the activation of microglia as well.1. When a metabolic injury occurs, such as the deprivation of oxygen, serum and glucose, the early process during the glial response is the secretion of S100B.7. The high concentrations of S100B cause neuronal death through nitric oxide release from astrocytes.5. The biological half-life of S100B approximates 30 minutes.
Besides peripheral blood, S100B can be found in cord blood, urine, cerebrospinal fluid (CSF), amniotic fluid, and with markedly higher concentrations than these, in milk.8. The S100B content in serum is lower than that in CSF.4. Many extracerebral sources contribute to the serum S100B content. Immunoassays and mRNA quantification have characterized other cells as S100B-expressing cells, particularly adipocytes, chondrocytes, lymphocytes, bone marrow cells, and melanoma cells. These data explain why controversy has arisen in recent years as to the origin of serum S100B and the involvement of brain damage, or not, in this release.4.
Glial fibrillary acidic protein (GFAP)
GFAP protein, a member of the intermediate filament family that provides support and strength to cells. Several molecules of GFAP protein bind together to form the main intermediate filament found in specialised brain cells called astrocytes. Astrocytes are star-shaped cells that support the functions of nerve cells in the brain and spinal cord (central nervous system). If the central nervous system is injured through trauma or disease, astrocytes react by rapidly producing more GFAP.
Although its function is not fully understood, GFAP protein is probably involved in controlling the shape and movement of astrocytes. The protein probably also plays a significant role in the interactions of astrocytes with other cells, which are required for the formation and maintenance of the insulating layer (myelin) that covers nerve cells. Additionally, GFAP protein may assist in maintaining the protective barrier that allows only certain substances to pass between blood vessels and the brain (blood-brain barrier).
SOMA MARKERS
Neurone Specific Enolase (NSE)
Neuron specific enolase (NSE) is an enzyme which catalyzes the conversion of 2-phosphoglycerate to phosphoenolpyruvate in the glycolytic pathway, and also the reverse reaction in gluconeogenesis. It is one of three mammalian enolases, which are also known as ENO1, ENO2, and ENO3 or alternately as enolase alpha, beta and gamma. NSE corresponds to ENO2 or enolase gamma and is heavily expressed in neuronal cells.
NSE is a 78 kDa glycolytic protein that is located primarily in the neuronal cytoplasm. It assists in the elevation of intraneural chloride concentrations during nerve excita- tion. NSE has been evaluated as a marker for mild traumatic brain injury in a number of studies. Similar to S-100, the sensitivity of NSE for mild traumatic brain injury is highly variable, ranging from 40%-89%.9, 10, 11, 12 Furthermore, elevated post-concussive levels of NSE do not appear to specifically predict persistent post-concussive symptoms.9, 10, 11, 12 Based on the available evidence, NSE does not have adequate sensitivity to assist with diagnosis of mild traumatic brain injuries, nor does it provide significant prognostic data related to mild traumatic brain injuries.
Since neurons require a great deal of energy, they are very rich in glycolytic enzymes such a GAPDH and NSE. Antibodies to this protein are therefore useful to identify neuronal cell bodies, developing neuronal lineage and neuroendocrine cells. Release of NSE from damaged neurons into CSF and blood has also been used as a biomarker of neuronal injury.13
Ubiquitin Carboxyl-Terminal Hydrolase Isoenzyme (UCH-L1)
The UCHL1 gene provides instructions for making an enzyme called ubiquitin carboxyl-terminal esterase L1. This enzyme is found in nerve cells throughout the brain. Ubiquitin carboxyl-terminal esterase L1 is probably involved in the cell machinery that breaks down (degrades) unneeded proteins. In cells, damaged or excess proteins are tagged with molecules called ubiquitin. Ubiquitin serves as a signal to move these unneeded proteins into specialized structures known as proteasomes, where the proteins are degraded. The ubiquitin-proteasome system acts as the cell's quality control system by disposing of damaged, misshapen, and excess proteins.
Although the exact function of ubiquitin carboxyl-terminal esterase L1 is not fully understood, it appears to have two types of enzyme activity. One of these, called hydrolase activity, removes and recycles ubiquitin molecules from degraded proteins. This recycling step is important to sustain the degradation process. The other enzyme function, known as ligase activity, links together ubiquitin molecules for use in tagging proteins for disposal.
AXON MARKERS
Neurofilament Light Polypeptide (NFL)
Neurofilaments are composed of neuron-specific intermediate filaments.16 Each intermediate filament consists of one light subunit (NFL) plus either a medium subunit (NFM) or a heavy subunit (NFH), arranged head-to-tail.16 High levels of NFL have been demonstrated in CSF samples obtained by lumbar puncture from amateur boxers with mild TBI after a bout.14,15 The magnitude of the rise in NFL is larger than that for total tau protein, which suggests that mild TBI affects the long myelinated axons in white matter to a greater extent than it affects the short nonmyelinated axons in the cortex.14,15 NFL in CSF seems to be the most sensitive fluid biomarker of axonal injury to date.14,15 Interestingly, the levels of NFL in CSF obtained by lumbar puncture from amateur boxers correlate positively with their exposure to head trauma, such as the number of hits to the head received, and subjective and objective estimates of the intensity of the fight.14,15
TAU
Tau proteins interact with tubulin to stabilize microtubules and promote tubulin assembly into microtubules. Six tau isoforms exist in brain tissue.
Hyperphosphorylation of the tau protein (tau inclusions), however, can result in the self-assembly of tangles of paired helical filaments and straight filaments, which are involved in the pathogenesis of Alzheimer’s disease and other Tauopathies.
Tau protein is a highly soluble microtubule-associated protein (MAP). In humans, these proteins are mostly found in neurons compared to non-neuronal cells.
One of tau’s main functions is to modulate the stability of axonal microtubules. Tau is not present in dendrites and is active primarily in the distal portions of axons where it provides microtubule stabilization but also flexibility as needed.
The tau gene locates on chromosome 17q21, containing 16 exons. Thus, in the human brain, the tau proteins constitute a family of six isoforms with the range from 352-441 amino acids.All of the six tau isoforms are present in an often hyperphosphorylated state in paired helical filaments from Alzheimer’s Disease brain.
In other neurodegenerative diseases such as TBI, the deposition of aggregates enriched in certain tau isoforms has been reported. When misfolded this otherwise very soluble protein can form extremely insoluble aggregates that contribute to a number of neurodegenerative diseases.
References
1.Rothermundt M, Peters M, Prehn JH, Arolt V. S100B in brain damage and neurodegeneration. Microsc Res Tech 2003; 60: 614-32.
2. Sedaghat F, Notopoulos A. S100 protein family and its application in clinical practice. Hippokratia 2008; 12: 198-204.
3. Drohat AC, Baldisseri DM, Rustandi RR, Weber DJ. Solution structure of calcium-bound rat S100B(betabeta) as determined by nuclear magnetic resonance spectroscopy. Biochemistry 1998; 37: 2729-40.
4. Gonçalves CA, Leite MC, Nardin P. Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin Biochem 2008; 41: 755-63.
5. Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 2001; 33: 637-68.
6. Sen J, Belli A. S100B in neuropathologic states: the CRP of the brain? J Neurosci Res 2007; 85: 1373-80.
7. Gerlach R, Demel G, König HG, Gross U, Prehn JH, Raabe A, et al. Active secretion of S100B from astrocytes during metabolic stress. Neuroscience 2006; 141: 1697-701.
8. Gazzolo D, Monego G, Corvino V, Bruschettini M, Bruschettini P, Zelano G, et al. Human milk contains S100B protein. Biochim Biophys Acta 2003; 1619: 209-12.
9. GeyerC,UlrichA,GräfeG,StachB,TillH.DiagnosticvalueofS100B and neuron-specific enolase in mild pediatric traumatic brain injury. J Neurosurg Pediatrics 2009;4:339-344.
10. deBoussardC,FredmanP,LundinA,AnderssonK,EdmanG,BorgJ. S100 in mild traumatic brain injury. Brain Inj 2004;18:671-683.
11. Berger R, Adelson PD, Pierce MC, Dulani T, Cassidy LD, Kochanek PM. Serum neuron-specific enolase, S-100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. 4. J Neurosurg 2005;103:61-68.
12. StålnackeBM,BjörnstigU,KarlssonK,SojkaP.One-yearfollow-upof mild traumatic brain injury: Post-concussion symptoms, disabilities and life satisfaction in relation to serum levels of S-100B and neurone-
13. Begaz, T., Kyriacou, D. N., Segal, J. and Bazarian, J. J. Serum biochemical markers for post-concussion syndrome in patients with mild traumatic brain injury. J. Neurotrauma 23:1201-1210 (2006).
14. Zetterberg, H. et al. Neurochemical aftermath of amateur boxing. Arch. Neurol. 63, 1277–1280 (2006)
15. Neselius, S. et al. CSF-biomarkers in Olympic boxing: diagnosis and effects of repetitive head trauma. PLoS ONE 7, e33606 (2012).
16. Liu, Q. et al. Neurofilament proteins in neurodegenerative diseases. Cell. Mol. Life Sci.61, 3057–3075 (2004).