Introduction
To date, the acute and chronic molecular effects of mild traumatic brain injury (TBI) have not been well studied or characterised and currently there are no validated diagnostic tests or biomarkers available to diagnose either the presence or recovery of the disease.
We have discovered a unique set of blood-based biomarkers, which are specific to TBI and possibly provide far superior diagnostic and prognostic power than any TBI test to this time. Our biomarkers comprise a panel of specific micro ribonucleic acids (miRNAs). The concentrations of these miRNAs in a patient’s blood are highly sensitive, and are regulated according to the patient’s level of TBI (ranging from no TBI to severe).
As a biomarker, miRNAs provide several unique characteristics:
- Cell, tissue and disease-specific.1-3.
- Small enough to cross the blood brain barrier (BBB) and enter the bloodstream
- Easily detected via modern test methods
- Do not require ‘normal’ baseline results (pre-TBI)
- Measure the ‘mechanism’ of pathology, rather than the end-stage
- ‘Quantify’ injury and therefore provide a prognostic as well as diagnostic tool
- Enable a Return to Play (RTP), battlefield, work, school, etc. trigger
This represents a significant advancement over previously available biomarkers and indeed TBI test methods.
miRNA
In recent years, blood and cerebrospinal fluid (CSF) biomarkers have emerged as possible tools to distinguish between the different pathophysiological processes after TBI.4. miRNAs, present in blood and CSF, are small, unique molecules that bind to messenger RNAs (mRNA) and inhibit the production of proteins. In many cases, miRNAs are upregulated in neuropathological conditions such as Alzheimer’s and Parkinson’s disease, leading to the original hypothesis that miRNAs could be important mediators of the profound molecular and cellular changes that occur after TBI in both the short and the long term.
We have found evidence that mTBI produces significant changes in specific miRNAs that are detectable in the blood, and it is believed that these miRNA are contributing to the pathology that occurs in the brain after injury.
Scientific and Clinical Standing
Studies highlight how the miRNA-based biomarkers in the serum and CSF could serve as promising diagnostic tools in TBI.5.
miRNAs have proven to be important mediators of the massive molecular and cellular changes that occur in both short and long-term periods post TBI. Their expression pattern alters significantly in response to TBI, and miRNAs play key roles in signal transduction, transcriptional regulation, mechanotransduction, proliferation, and differentiation initiated by TBI. Mechanical force is one of the important determinants in TBI, and Weber and colleagues proposed how mechanical shear stresses could modulate homeostasis through miRNA regulation of target molecules.6. They conducted a study on human endothelial cells subjected to unidirectional shear stress, and data showed 13 microRNAs with increased expressions. They concluded shear stress could regulate miRNA expression in endothelial cells, thereby suggesting potential roles of miRNAs on mechanotransduction and injury response.7. In addition, Redell and colleagues firstly reported plasma miRNA levels altered in TBI patients compared to age-, gender-, and race-matched healthy volunteers.8. Based on quantitative analysis of RT-PCR data, they suggested that plasma miRNA could be used as early circulating markers.
Our Findings
We have found evidence that mTBI produces significant changes in specific miRNAs that are detectable in the plasma, and it is possible that these miRNA are contributing to the pathology that occurs in the brain after injury.
Indeed, our panel of miRNAs linked to inflammatory cascade may have utility in diagnosis of TBI (Table & Figure below). We are actively seeking opportunities to cross-validate our miRNA panel with secondary measures of the injury performed in clinical studies.
Detail your services
Display real testimonials
Display real testimonials
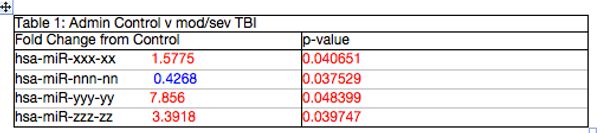
Four miRNAs are altered in moderate to severe TBI. Ten moderate to severe TBI subjects with a GCS<12 had blood drawn upon entry to the emergency department. The results of their initial draw are shown in table 1. Clearly, there are significant differences in miRNA present in the moderate to severe TBI group. Of interest, these are the same miRNA that were altered in a subset of the mild TBI football and soccer players presented in figure below.
Display real testimonials
Display real testimonials
Display real testimonials
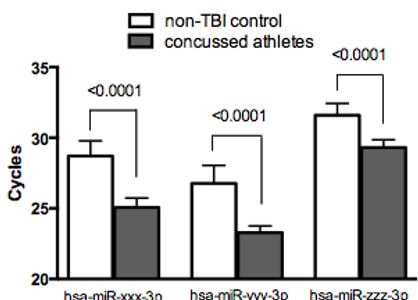
Three miRNAs shown are significantly altered following repeated mild TBI in male football and female soccer players. Seventy-seven athletes had blood drawn prior to the beginning of their respective seasons. Of this group, 17 individuals self-reported having persistent symptoms following a concussion (headaches, difficulty concentrating, and insomnia). However, none of the 17 athletes had suffered a concussion in the 6 months prior to testing. At the time of the blood draw none of the athletes had any TBI associated symptoms present. Results from our analysis clearly show these 17 athletes were significantly different from other athletes or non-TBI controls. These results suggest a sub-population of athletes (22%) may be vulnerable to mTBI and may experience long-term effects that occur at a molecular level. Athletes (13 males and 4 females) mean athlete age=22 years. Non-TBI controls (6 males, 9 females) mean age of 31. Two-tailed T-test was used for each comparison.
Four miRNAs are altered in moderate to severe TBI. Ten moderate to severe TBI subjects with a GCS<12 had blood drawn upon entry to the emergency department. The results of their initial draw are shown in table 1. Clearly, there are significant differences in miRNA present in the moderate to severe TBI group. Of interest, these are the same miRNA that were altered in a subset of the mild TBI football and soccer players presented in figure below.

Three miRNAs shown are significantly altered following repeated mild TBI in male football and female soccer players. Seventy-seven athletes had blood drawn prior to the beginning of their respective seasons. Of this group, 17 individuals self-reported having persistent symptoms following a concussion (headaches, difficulty concentrating, and insomnia). However, none of the 17 athletes had suffered a concussion in the 6 months prior to testing. At the time of the blood draw none of the athletes had any TBI associated symptoms present. Results from our analysis clearly show these 17 athletes were significantly different from other athletes or non-TBI controls. These results suggest a sub-population of athletes (22%) may be vulnerable to mTBI and may experience long-term effects that occur at a molecular level. Athletes (13 males and 4 females) mean athlete age=22 years. Non-TBI controls (6 males, 9 females) mean age of 31. Two-tailed T-test was used for each comparison.
Our published studies till now;
1. Mitra B, et al. Plasma micro-RNA biomarkers for diagnosis and prognosis after traumatic brain injury: A pilot study. J Clin Neurosci (2017), http://dx.doi.org/10.1016/j.jocn.2016.12.009
2. Diane M, et al. Multiple mild traumatic brain injury in the rat produces persistent pathological alterations in the brain Exp. Neu (2017), http://dx.doi.org/10.1016/j.expneurol.2017.07.015
3. Rau T, et al. Efficacy of a repeat testing protocol for cognitive fatigue assessment: a preliminary study in postconcussive syndrome participants. Concussion (2017) https://www.futuremedicine.com/doi/suppl/10.2217/cnc-2017-0002
4. Mitra B, et al. Micro-RNA levels and symptom profile after mild traumatic brain injury: A longitudinal cohort study. J Clin Neuroscience (2021), https://doi.org/10.1016/j.jocn.2021.11.021
5. Mitra B, et al. MicroRNA biomarkers for diagnosis of mild traumatic brain injury and prediction of persistent symptoms: A prospective cohort study. J Clin Neuroscience (2023), https://doi.org/10.1016/j.jocn.2023.07.011

References
1. Bartels CL, Tsongalis GJ. MicroRNAs: novel biomarkers for human cancer. Clinical chemistry 2009;55(4):623-31.
2. Vemuganti R. The microRNAs and stroke: no need to be coded to be counted. Translational stroke research 2010;1(3):158-60.
3. Ye Y, Perez-Polo JR, Qian J, et al. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiological genomics 2011;43(10):534-42.
4. Mrozek S, Dumurgier J, Citerio G, et al. Biomarkers and acute brain injuries: interest and limits. Critical Care 2014;18(2):220-20.
5. Bhalala O.G., Srikanth M., Kessler J.A. The emerging roles of microRNAs in CNS injuries. Nat. Rev. Neurol. 2013;9:328–339. doi: 10.1038/nrneurol.2013.67.
6. Weber M., Baker M.B., Moore J.P., Searles C.D. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem. Biophys. Res. Commun. 2010;393:643–648. doi: 10.1016/j.bbrc.2010.02.045.
7. Saugstad J.A. MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. J. Cereb. Blood Flow Metab. 2010;30:1564–1576. doi: 10.1038/jcbfm.2010.101.
8. Redell J.B., Moore A.N., Ward N.H., Hergenroeder G.W., Dash P.K. Human traumatic brain injury alters plasma microRNA levels. J. Neurotrauma.2010;27:2147–2156. doi: 10.1089/neu.2010.1481.